Chimeric antigen receptor (CAR) T-cell therapy is a type of regenerative immunotherapy developed to aid in treating various blood cancers. CAR-T therapies use a patient’s own immune system to help fight cancer. This revolutionary therapy introduces genetically-enhanced living T-cells, which are white blood cells that are part of the immune system. T cells are taken from the patient’s blood and altered in a lab by adding a gene for a receptor, which helps the T cells attach to a specific cancer cell antigen. Once the cells have been modified, they must be preserved to maintain their therapeutic effects. The manufacturers prefer cryopreservation as their method to keep cells alive until they can be administered back to the patient[20]. The CAR-T cells are then given back to the patient by infusion to locate and destroy cancer cells.
The work on CAR-T cells traces back to 1989 when a trio of Israeli immunologists worked on the expression of chimeric genes of T-cell receptors. Gideon Gross, Tova Waks, and Zelig Eshhar published a monumental experiment where they utilized genomic vectors with specific chimeric gene segments that would express new receptors on the cell[1]. These receptors provided T-cells with a new specificity to act like an antibody and effectively recognize antigens at the surface. This experiment hallmarked engineered lymphocytes to improve killer T-cell functions.
There are four generations of CAR-T cell development. Each generation marks significant improvements to the design of the receptors, with each iteration enhancing the CARs. The first generation of CARs was not able to sustain T-cell responses. The early 2000s brought along the second generation of CARs, which worked in pairs to increase activation and signaling of the receptors that improved T-cell responses and cell viability. These second-generation CARs T cells were able to survive, multiply, and kill cancer cells in the lab, establishing the feasibility of CAR-T cell therapy[19]. The third generation combined the coupled power of the different signaling domains into one. As recent as 2017, the fourth generation has additional genetic modifications for tumor-killing activity[2]. Out of all the generations thus far, the approved therapy in the market is based on the second generation of CARs.
Around the same time, the fourth generation of CAR-T cells was in development, the U.S. Food and Drug Administration (FDA) approved the first immunotherapy utilizing this technology. The first CAR-T therapy approved by the FDA was Kymriah (generic name – Tisagenkecleucel), a treatment for patients over 25 years of age with relapsing acute lymphoblastic leukemia (ALL)[3]. The initial approval letter by the FDA also included a voucher for rare pediatric disease applications, which allowed this therapy to be available for pediatric cases on minimal bases[4].
There are currently six FDA-approved therapies, including Kymriah, for various types of lymphoma and myeloma. Kymriah’s demographic reach expanded in the following years. In 2018, the FDA approved this therapy to include adult patients with large B-cell lymphoma; in 2022, the FDA approved this therapy for patients with follicular lymphoma[5,6]. In all three different approvals of Kymriah, cancer must be relapsed, which means that the patient was in remission or cancer-free, and cancer came back or refractory, which means the cancer is unresponsive to treatment.
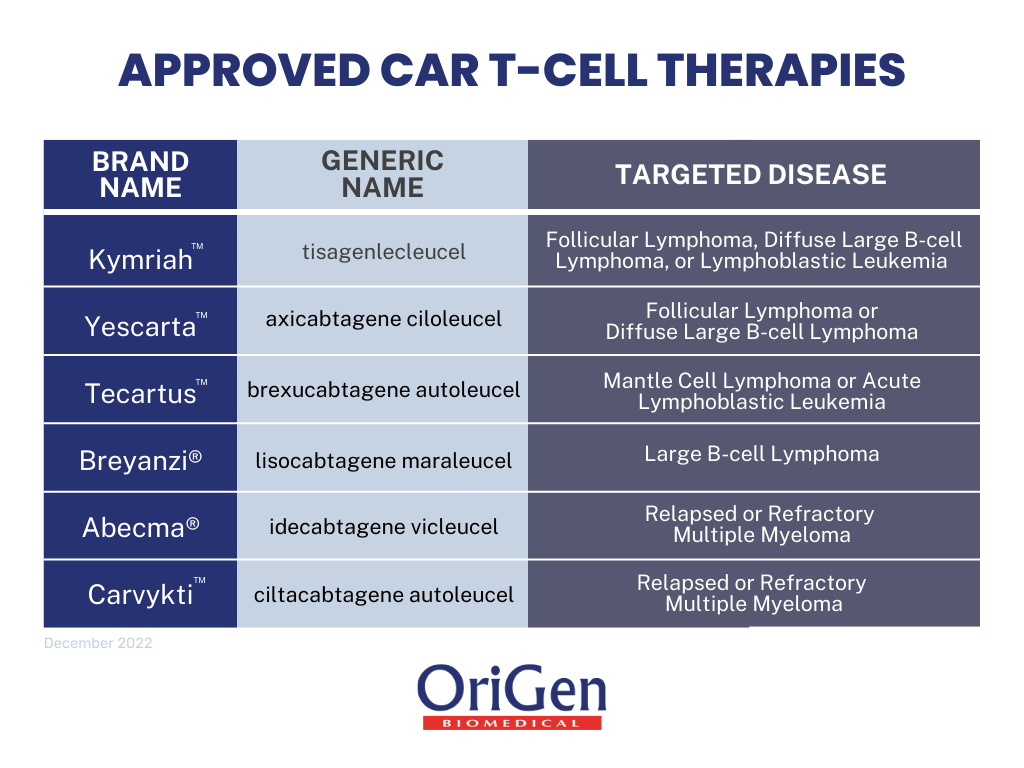
The second approved therapy was Yescarta (generic name – axicabtagene ciloleucel) in 2017. Initially, the FDA approved this treatment for adult large B-cell lymphoma patients after at least two unsuccessful lines of systemic therapy[7]. In 2021, the FDA expanded this therapy to include follicular lymphoma[8]. There was a change in the qualifications for this treatment in 2022, where the updated approval indicated that this treatment could be used by adult patients with large B-cell lymphoma, where cancer is refractory to the first line of therapy or if it relapses within a year of the first line of therapy[9].
The subsequent therapy approved was Tecartus (generic name – brexucabtagene autoleucel) in 2020. The FDA approved this therapy for adult patients with mantle cell lymphoma (MCL)[10]. It was later approved for B-cell precursor acute lymphoblastic leukemia (ALL)[11]. Following Tecartus, the FDA approved Breyanzi (generic name- lisocabtagene maraleucel) in early 2021 for adult patients[12]. As with the rest of the CAR-T therapies, the treatment is for relapsed or refractory cancer, specifically large B-Cell lymphoma, after two lines of unsuccessful therapy. In June of 2022, Breyanzi had an approved change to allow adult patients to use the therapy with relapse or refractory disease within a year of the first line of chemoimmunotherapy, as well as patients that are not eligible for hematopoietic stem cell transplantation[13].
The following two approved therapies are for relapsed or refractory multiple myeloma, a cancer of plasma cells. Abecma (generic name – idecabtagene vicleucel) was approved in March 2021 for patients with multiple myeloma after at least four lines of therapy[14]. The indications for this treatment are more specific, as the prior lines of therapy must include an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody. In addition to Abecma, Carvykti (ciltacabtagene autoleucel) was approved in February of 2022, making it the most recently approved CAR-T therapy[15]. Carvykti has the same indications as Abecma for patient eligibility.
As successful as it is, CAR-T cell research continues to grow to improve immunotherapy technology. In December 2019, there were 284 clinical trials for CAR-T cell therapies, and as of October 2022, that number tripled to over 800 trials[16,17]. Ongoing research targets the use of antigens to target solid tumors since current approved therapies are for blood cancers only[18].
OriGen Biomedical is honored to support CAR-T technology and help turn cancer patients into cancer survivors. We are proud to be recognized as the industry-preferred choice for single-use disposables within cell and gene therapy, including CAR-T therapy. At OriGen, we believe that our primary responsibility is to the patients. We offer a wide range of medical devices that are successfully employed to treat blood cancers throughout the CAR-T process.
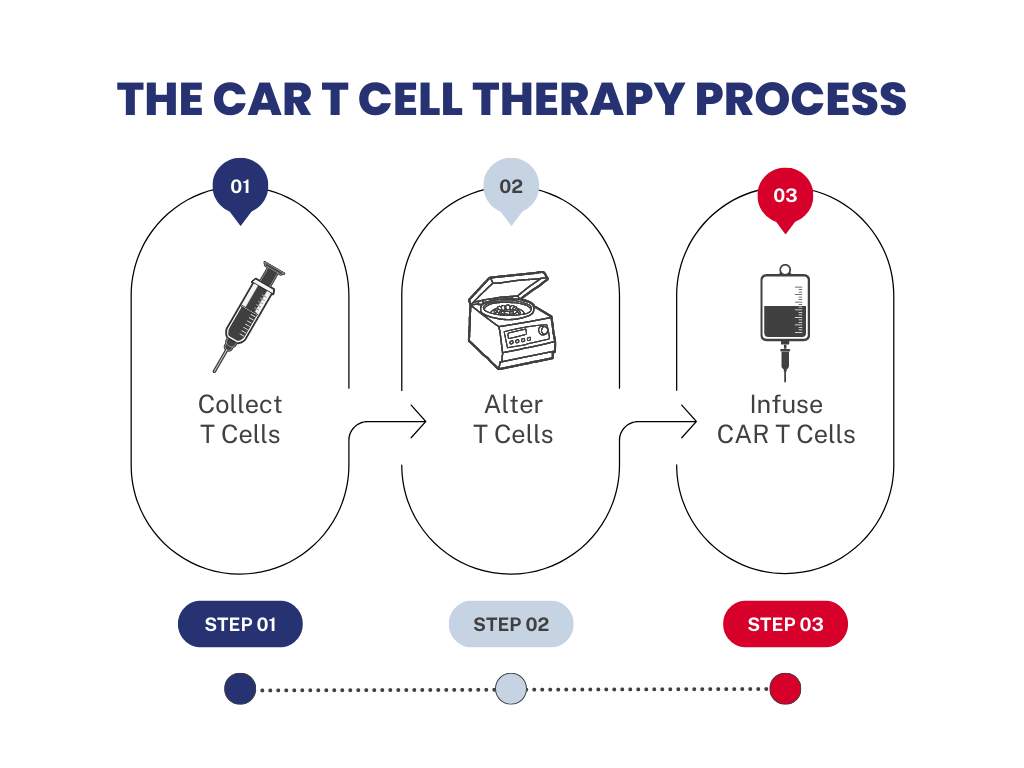
- The CAR-T cell therapy process starts with drawing blood from a patient. T cells are collected through a process called apheresis where the patient’s blood is separated into different cell types. White blood cells, including T-cells, are collected and then the rest of the blood is returned to the patient’s body. These white blood cells are collected using our accessories for closed system processing and stored within a cryopreservation bag, CryoStore Freezing Bags, that is frozen prior to transport. Cells are kept viable and functional through cryopreservation while cells are transported between different sites throughout the process.
- The cryopreservation bags are then sent to the manufacturing site for modification. At the manufacturing site, CARs are added to T-cells to reprogram them to find cancer cells using syringes and cultured in smaller volume cell culture bags, PermaLife® Cell Culture Bags.
- Next, modified T-cell expansion occurs until the required amount for an appropriate dose is available for therapy, which can vary from patient to patient using our mid-to-large volume PermaLife Cell Culture Bags and syringes.
- Once manufactured, the final cell product is stored in a cryopreservation bag, CryoStore, and cryopreserved with a cryopreservation solution, Cryopur® Solutions. Various accessories, like our manifold sets, can be used for both open- and closed-system processing. The cryopreserved final product is shipped back to the patient at the clinical site where the CAR-T cells are thawed and returned to the patient via intravenous (IV) infusion. The infused cells will then multiply, bind to cancer cells to kill them, and ultimately provide treatment.
Scientists are currently trying to determine if the CAR-T treatment eliminates all cancer in that initial treatment or if it continuously finds and destroys the cancer as it reappears throughout the remainder of the patient’s life. Just this year, more than 10 years after the first CAR-T treatments were given, leading researchers in the CAR-T field felt confident to use the word ‘cured.’ The term ‘cure’ is a big deal in the world of oncology, but year after year recipients of CAR-T patients remain cancer free – which is ultimately the important part.[21]
At OriGen, we believe that our primary responsibility is to the patients and we are incredibly proud to support CAR-T cell therapies. Together we are making a difference in the fight against cancer.