Standard Reference
Symbol Title
Explanatory Text

ISO 15223-1, Clause 5.1.1
Manufacturer
Indicates the medical device manufacturer.

ISO 15223-1, Clause 5.7.7
Medical Device
Indicates the item is a medical device.

ISO 15223-1, Clause 5.1.2
Authorized Representative in the European Community / European Union
Indicates the Authorized representative in the European Community / European Union.

765/2008/EC 768/2008/EC MDD 93/42/EEC Articles 4,11,12,17, Annex II ) RED 2014/53/EU (Articles 19, 20, Annex II)
CE Mark
Indicates the device meets the essential requirements of all relevant European Medical Device Directives.

ISO 15223-1, Clause 5.1.6
Catalogue or Model Number
Indicates the manufacturer’s catalogue number so that the medical device can be identified.

ISO 15223-1, Clause 5.1.5
Batch Code or Lot Number
Indicates the manufacturer’s batch code so that the batch or lot can be identified.

ISO 15223-1, Clause 5.1.4
Use-By Date
Indicates the date after which the medical device is not to be used.

ifu.origen.com
ISO 15223-1, Clause 5.4.3
Consult Instructions for Use or Consult Electronic Instructions for Use
Indicates the need for the user to consult the instructions for use.

<yellow>
ISO 15223-1, Clause 5.4.4
Caution
Indicates that caution is necessary when operating the device or control close to where the symbol is placed, or that the current situation needs operator awareness or operator action in order to avoid undesirable consequences.
US FDA Guidance: Alternative to Certain Prescription Device Labeling Requirements
Prescription Only
Caution: Federal law restricts this device to sale by or on the order of a licensed healthcare practitioner.

ISO 15223-1, Clause 5.3.4
Keep Dry
Indicates a medical device that needs to be protected from moisture.

ISO 15223-1, Clause 5.2.1
Sterile
Indicates a medical device that has been subjected to a sterilization process.

ISO 15223-1, Clause 5.2.3
Sterilized Using Ethylene Oxide
Indicates a medical device that has been sterilized using ethylene oxide.

ISO 15223-1, Clause 5.2.9
Sterile Fluid Path
Indicates the presence of a sterile fluid path within the medical device in cases when other parts of the medical device, including the exterior, might not be supplied sterile.

ISO 15223-1, Clause 5.2.4
Sterilized Using Irradiation
Indicates a medical device that has been sterilized using irradiation.

ISO 15223-1, Clause 5.2.2
Sterilized Using Aseptic Processing Techniques
Indicates a medical device that has been manufactured using accepted aseptic techniques.

ISO 15223-1, Clause 5.2.6
Do Not Resterilize
Indicates a medical device that is not to be resterilized.

ISO 15223-1, Clause 5.2.8
Do Not Use if Package is Damaged and Consult Instructions for Use
Indicates that a medical device that should not be used if the package has been damaged or opened and that the user should consult the instructions for use for additional information.
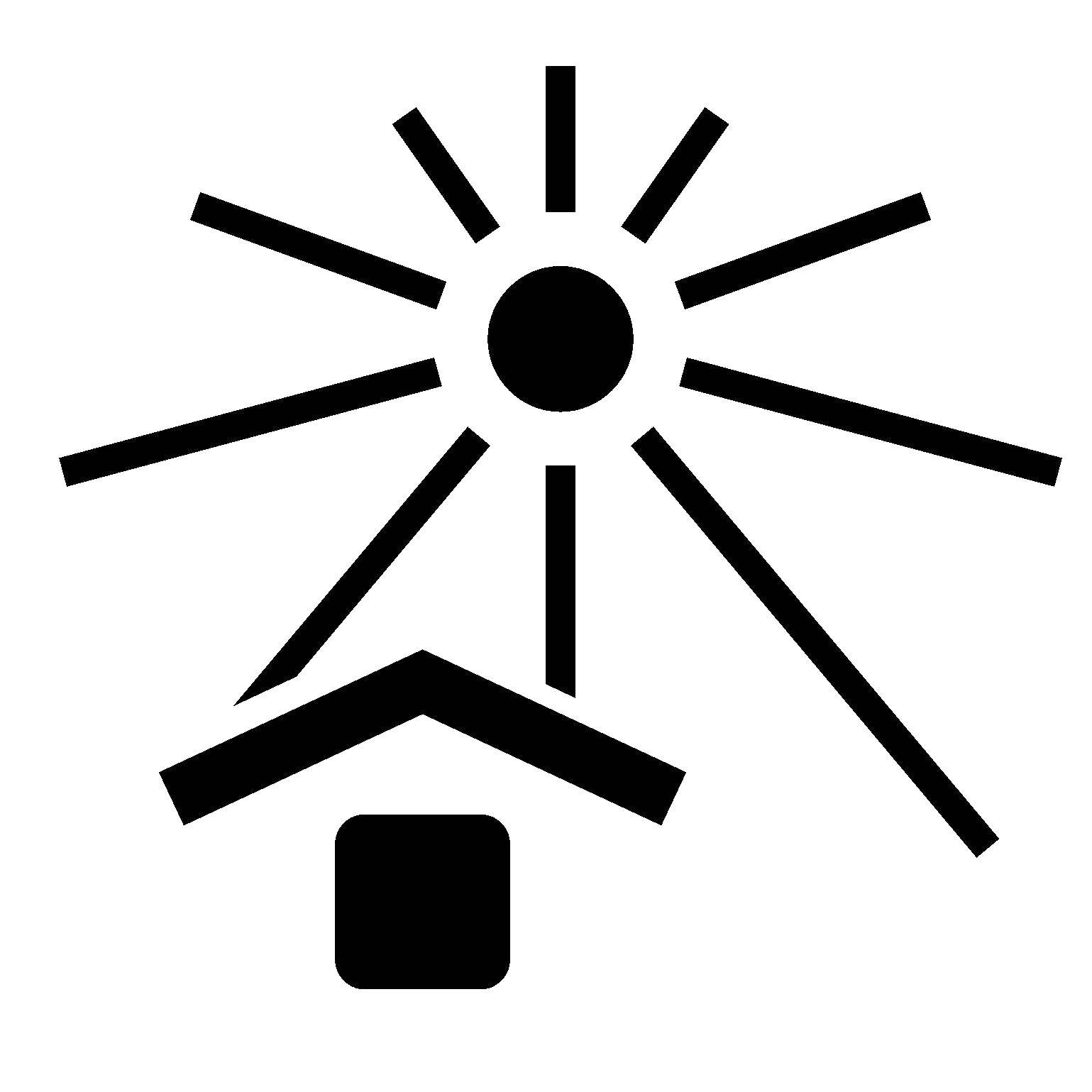
ISO 15223-1, Clause 5.3.2
Keep Away from Sunlight
Indicates a medical device that needs protection from light sources.

ISO 15223-1, Clause 5.4.2
Do Not Reuse
Indicates a medical device that is intended for one single use only.

ISO 15223-1, Clause 5.6.3
Non-Pyrogenic
Indicates a medical device that is non-pyrogenic.

ISO 15223-1, Clause 5.6.2
Fluid Path
Indicates the presence of a fluid path.

ISO 15223-1, Clause 5.3.7
Storage Temperature Range
Indicates the temperature limits to which the medical device can be safely exposed.
Non-harmonized Symbol
Quantity
Indicates the number of units in the associated packaging.

ISO 15223-1, Clause 5.3.1
Fragile, Handle with Care
Indicates a medical device that can be broken or damaged if not handled carefully.

ISO 3826-2, Clause 4.3.1
Blood or Blood Component Container
On medical devices or blood process application: to indicate that the processing or final container is used for the purpose of whole blood or blood component storage.

Non-harmonized Symbol
Freezing Bag
Indicates that the product is intended for freezing at cryogenic temperatures. Values displayed to the right of the image indicate the validated freeze volume.

ISO 3826-2, Clause 4.4.7
Processing Container
On medical devices or blood process application: to indicate that the processing of final container is used for the achievement of a process.

ISO 3826-2
Buffy Coat Container
On medical devices or blood process application: to indicate that the container is used for the purpose of final or temporary storage of buffy coat. (Buffy coat is the combination of leukocyte and platelets obtained after centrifugation of whole blood.)

ISO 15223-1:2021, Clause 5.7.10
Unique Device Identifier
Identifies the Unique Device Identifier including the Automatic identification and data capture (AIDC) and human-readable information.

ISO 15223-1, Clause 5.2.14
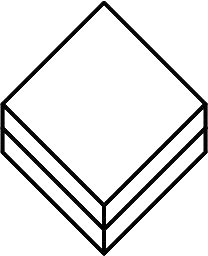
Single Sterile Barrier System with Protective Packaging Outside
Indicates that the medical device has a single sterile barrier system with protective packaging outside.
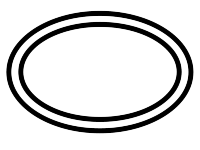
ISO 15223-1, Clause 5.2.12
Double Sterile Barrier System
Indicates that the medical device has a double sterile barrier system.
